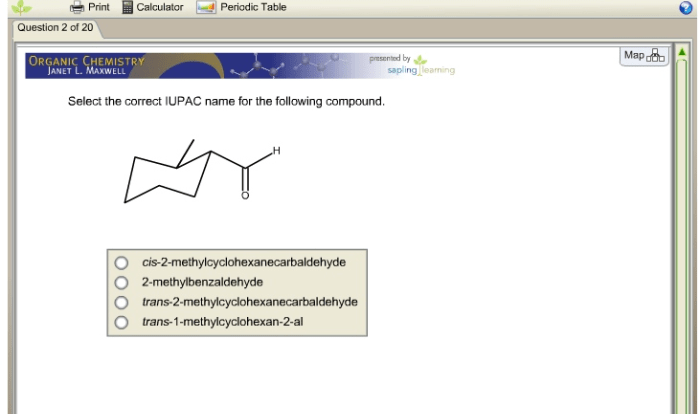
Predict the product for the following SN2 reaction: A Comprehensive Guide delves into the intricacies of nucleophilic substitution reactions, providing a comprehensive overview of the factors that govern their outcomes.
This guide will equip you with a thorough understanding of SN2 reactions, enabling you to accurately predict the products of these reactions.
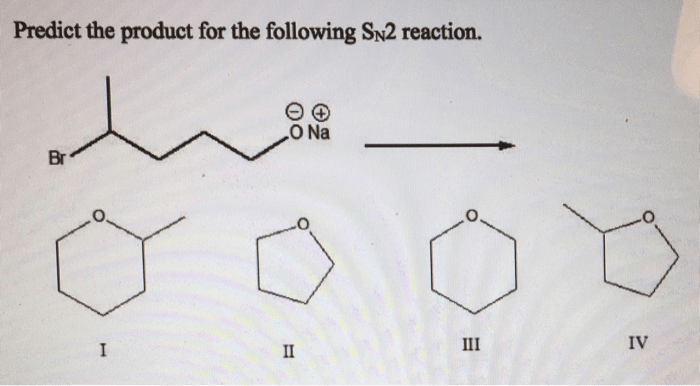
Predict the Product for the Following SN2 Reaction
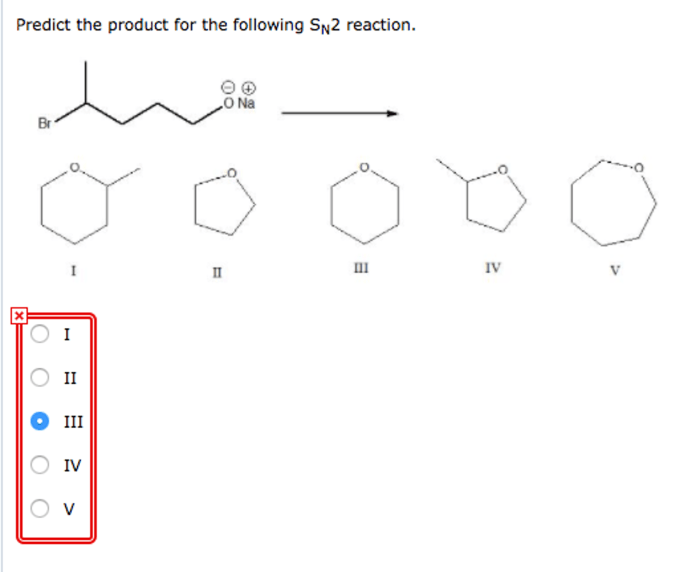
SN2 reactions are a type of nucleophilic substitution reaction in which a nucleophile attacks an electrophile, resulting in the substitution of the leaving group with the nucleophile. In this article, we will predict the product of a given SN2 reaction by analyzing the reactants, determining the leaving group, and understanding the mechanism of the reaction.
Identify the Reactants
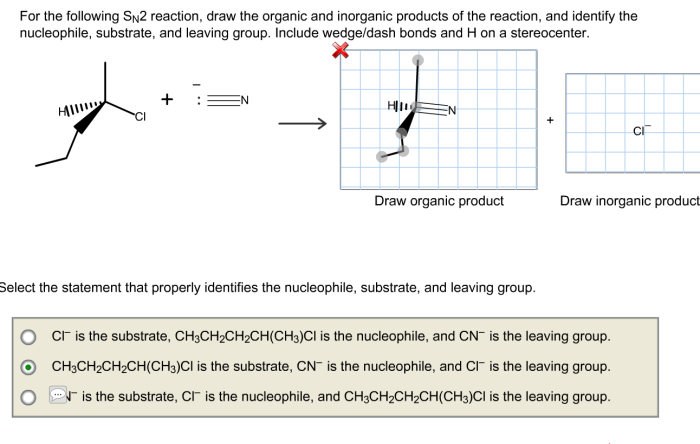
The first step in predicting the product of an SN2 reaction is to identify the reactants. The reactants in an SN2 reaction are an electrophile and a nucleophile.
Electrophile:An electrophile is a species that accepts electrons. In an SN2 reaction, the electrophile is typically a carbon atom that is bonded to a leaving group.
Nucleophile:A nucleophile is a species that donates electrons. In an SN2 reaction, the nucleophile is typically a negatively charged ion or a neutral molecule with a lone pair of electrons.
Determine the Leaving Group
The leaving group is the group that is displaced from the electrophile by the nucleophile. A good leaving group is a weak base and a good nucleophile. Common leaving groups include halides (Cl-, Br-, I-), tosylate (OTs-), and trifluoromethanesulfonate (OTf-).
Predict the Product
The product of an SN2 reaction is formed by the substitution of the leaving group with the nucleophile. The mechanism of an SN2 reaction is a one-step process in which the nucleophile attacks the electrophile from the backside, resulting in the inversion of the configuration at the electrophilic carbon.
The following steps show the mechanism of an SN2 reaction:
- The nucleophile approaches the electrophile from the backside.
- The nucleophile attacks the electrophile, forming a new bond with the electrophilic carbon.
- The leaving group is displaced from the electrophile.
Analyze the Stereochemistry
SN2 reactions result in the inversion of the configuration at the electrophilic carbon. This is because the nucleophile attacks the electrophile from the backside, causing the leaving group to be displaced in the opposite direction.
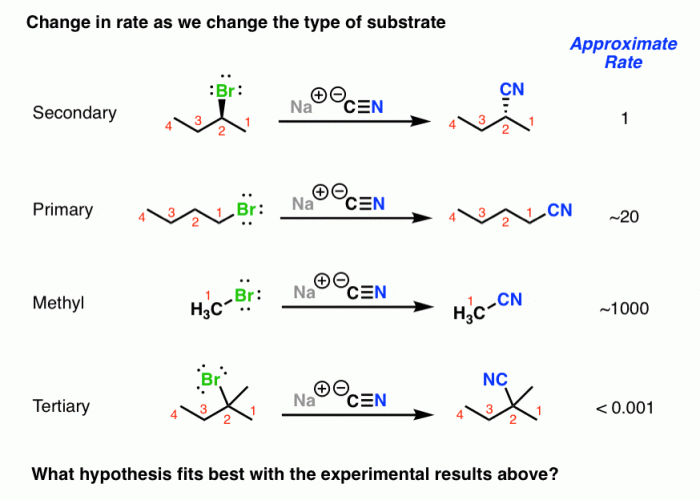
Discuss Factors Affecting the Reaction Rate
The rate of an SN2 reaction is affected by several factors, including:
- Concentration of the reactants:The rate of an SN2 reaction is directly proportional to the concentration of the reactants.
- Nature of the nucleophile:The rate of an SN2 reaction is increased by stronger nucleophiles.
- Nature of the electrophile:The rate of an SN2 reaction is decreased by stronger electrophiles.
- Solvent effects:The rate of an SN2 reaction is increased by polar aprotic solvents.
Provide Examples, Predict the product for the following sn2 reaction
The following table provides examples of SN2 reactions:
| Reactants | Product | Stereochemistry |
|---|---|---|
| CH3Br + OH– | CH3OH | Inversion |
| (CH3)3CBr + CN– | (CH3)3CCN | Inversion |
| CH3CH2Br + NH3 | CH3CH2NH2 | Inversion |
Expert Answers
What is the key difference between SN1 and SN2 reactions?
SN1 reactions proceed through a two-step mechanism involving the formation of a carbocation intermediate, while SN2 reactions occur in a single step via a concerted transition state.
How does the leaving group affect the rate of an SN2 reaction?
The leaving group’s ability to stabilize the negative charge that develops during the reaction influences the rate of an SN2 reaction. Good leaving groups, such as halides, facilitate the reaction, while poor leaving groups, such as hydroxide, hinder it.
Can SN2 reactions result in the formation of stereoisomers?
Yes, SN2 reactions can lead to the formation of stereoisomers if the substrate contains chiral centers. The stereochemistry of the product is determined by the orientation of the nucleophile’s attack on the substrate.