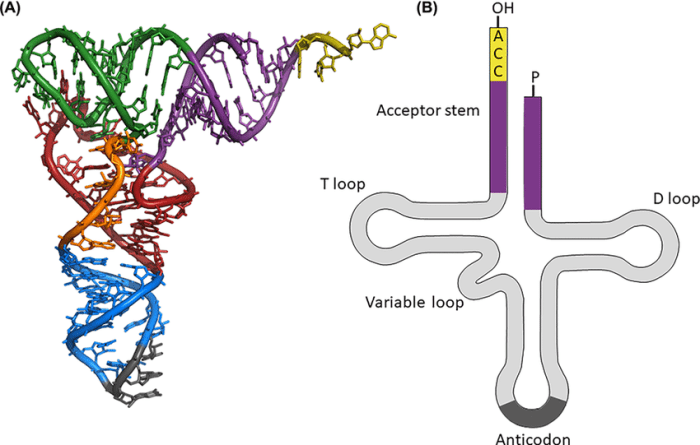
Label the structural features of the yeast phenylalanine trna – Labeling the structural features of the yeast phenylalanine tRNA unveils a complex and fascinating molecular architecture. This tRNA molecule, responsible for decoding the genetic code during protein synthesis, exhibits a remarkable array of structural elements, each playing a crucial role in its function.
This comprehensive guide delves into the intricate details of these features, providing a deeper understanding of their significance in tRNA biology.
The tRNA molecule, a cloverleaf-shaped RNA, comprises several distinct regions, including the anticodon loop, TψC loop, D loop, variable loop, acceptor stem, T arm, dihydrouridine loop, and others. Each of these regions possesses a unique sequence and structure, contributing to the overall stability, recognition, and specificity of the tRNA molecule.
Yeast Phenylalanine tRNA Structure: Label The Structural Features Of The Yeast Phenylalanine Trna
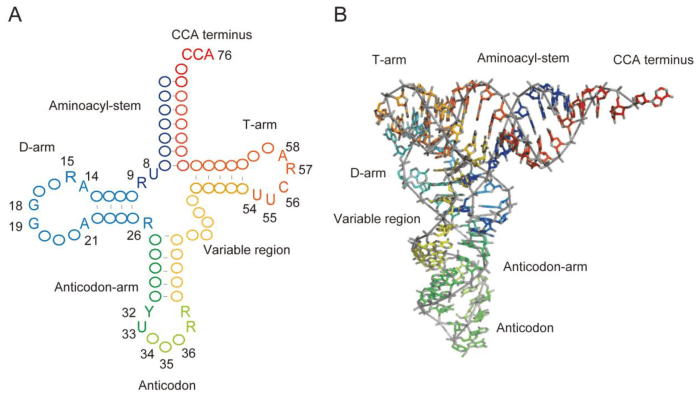
Yeast phenylalanine tRNA (Phe-tRNA) is a small RNA molecule that plays a crucial role in protein synthesis. It has a characteristic cloverleaf structure composed of four loops and three stems.
Anticodon Loop
The anticodon loop, located at the top of the cloverleaf, contains three nucleotides that are complementary to the codon on the messenger RNA (mRNA). The sequence of the anticodon loop in Phe-tRNA is 5′-UUC-3′, which recognizes and binds to the codon 5′-AAA-3′ on the mRNA.
TψC Loop
The TψC loop, located on the right side of the cloverleaf, is characterized by the presence of a modified nucleotide, pseudouridine (ψ), and a cytidine (C). The TψC loop is involved in tRNA stability and interacts with proteins that assist in tRNA folding and function.
D Loop
The D loop, located at the bottom of the cloverleaf, is characterized by a sequence of four nucleotides, including a dihydrouridine (D). The D loop is involved in tRNA folding and recognition by proteins involved in protein synthesis.
Variable Loop
The variable loop, located on the left side of the cloverleaf, varies in sequence among different tRNA species. It is involved in tRNA identity and specificity, helping to ensure that the correct tRNA is selected for each codon.
Acceptor Stem
The acceptor stem, located at the bottom of the cloverleaf, forms a double helix with seven base pairs. It contains the 3′-CCA sequence at its end, which is the site of amino acid attachment.
T Arm, Label the structural features of the yeast phenylalanine trna
The T arm, located at the top of the cloverleaf, is a single-stranded region that contains a conserved thymidine (T) nucleotide. The T arm plays a role in tRNA folding and stability.
Dihydrouridine Loop
The dihydrouridine loop, located on the right side of the cloverleaf, contains a modified nucleotide, dihydrouridine (D). The dihydrouridine loop is involved in tRNA stability and recognition by proteins involved in protein synthesis.
Detailed FAQs
What is the function of the anticodon loop in tRNA?
The anticodon loop, located at the top of the tRNA molecule, contains a three-nucleotide sequence that is complementary to a specific codon on the mRNA. This complementarity allows the tRNA to recognize and bind to the correct codon, ensuring the correct amino acid is incorporated into the growing polypeptide chain during protein synthesis.
How does the D loop contribute to tRNA folding?
The D loop, located at the bottom of the tRNA molecule, plays a crucial role in stabilizing the tRNA’s tertiary structure. It interacts with other regions of the tRNA, including the TψC loop and the variable loop, to form a compact and functional molecule.
What is the significance of the dihydrouridine loop in tRNA stability?
The dihydrouridine loop, located near the anticodon loop, contains a modified uridine nucleotide that contributes to the stability of the tRNA molecule. This modification protects the tRNA from degradation by enzymes and helps maintain its structural integrity during protein synthesis.