Welcome to our in-depth exploration of modern marvels corrosion and decomposition worksheet answers, where we delve into the fascinating world of material degradation and preservation. This comprehensive guide will provide you with a thorough understanding of the processes involved in corrosion and decomposition, empowering you to safeguard the longevity of modern structures and materials.
Corrosion and decomposition are pervasive forces that can significantly impact the integrity and lifespan of our built environment. Understanding the mechanisms behind these processes is crucial for developing effective strategies to prevent and mitigate their damaging effects. This worksheet delves into the intricacies of corrosion and decomposition, providing valuable insights into their causes, consequences, and potential solutions.
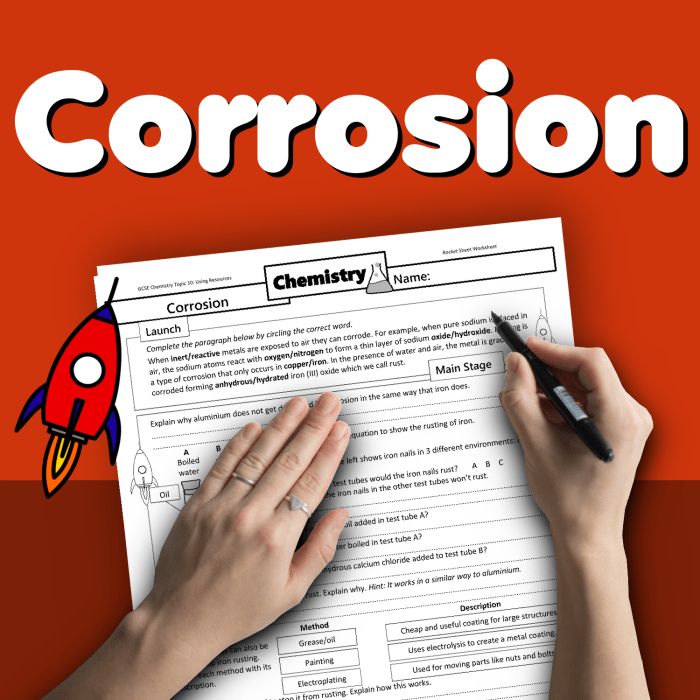
1. Corrosion and Decomposition Definitions: Modern Marvels Corrosion And Decomposition Worksheet Answers
Corrosion is the deterioration of a material, typically a metal, due to a chemical reaction with its environment. Decomposition is the breakdown of a material into simpler substances.
Corrosion involves the loss of electrons from the metal, leading to the formation of metal ions. Decomposition, on the other hand, can occur through various processes, such as hydrolysis, oxidation, or enzymatic reactions.
Examples of corrosion include the rusting of iron and the tarnishing of silver. Decomposition examples include the spoilage of food and the decay of organic matter.
2. Factors Influencing Corrosion and Decomposition

Environmental Factors
- Temperature: Higher temperatures accelerate both corrosion and decomposition.
- Humidity: High humidity promotes corrosion by providing moisture for electrochemical reactions.
- Exposure to Chemicals: Corrosive chemicals, such as acids and bases, can accelerate corrosion.
Material Properties
- Reactivity: Metals with high reactivity, such as iron, are more susceptible to corrosion.
- Surface Area: Materials with a larger surface area are more prone to corrosion.
- Protective Coatings: Coatings, such as paint or zinc, can protect materials from corrosion.
Design and Maintenance Practices
- Design: Proper design can minimize exposure to corrosive environments.
- Maintenance: Regular maintenance, such as cleaning and repainting, can extend the lifespan of materials.
3. Methods for Preventing Corrosion and Decomposition
Protective Coatings
- Paint: Paint provides a barrier against moisture and corrosive chemicals.
- Zinc Coatings: Zinc sacrifices itself to protect steel from corrosion.
- Polymers: Polymers, such as epoxy resins, can protect materials from chemical attack.
Sacrificial Anodes
- Galvanic Anodes: These anodes, made of a more reactive metal than the protected material, corrode instead of the protected material.
- Impressed Current Cathodic Protection: This method uses an external power source to protect the material from corrosion.
Corrosion Inhibitors
- Chemical Inhibitors: These chemicals form a protective layer on the metal surface.
- Organic Inhibitors: These inhibitors absorb on the metal surface and block the corrosion process.
4. Case Studies of Corrosion and Decomposition
Case Study: Golden Gate Bridge
The Golden Gate Bridge has been affected by corrosion due to exposure to salt water and high humidity. The bridge has been protected using a combination of paint, cathodic protection, and regular maintenance.
Case Study: Statue of Liberty
The Statue of Liberty has been affected by decomposition due to exposure to acid rain and pollution. The statue has been restored using a combination of chemical cleaning and the application of a protective coating.
5. Future Trends in Corrosion and Decomposition Prevention

Emerging Technologies
- Self-Healing Materials: These materials can repair themselves when damaged, reducing the need for maintenance.
- Nanotechnology: Nanotechnology-based coatings can provide enhanced protection against corrosion and decomposition.
Research Advancements, Modern marvels corrosion and decomposition worksheet answers
- Development of New Corrosion Inhibitors: Research is ongoing to develop more effective corrosion inhibitors.
- Understanding Corrosion Mechanisms: Ongoing research aims to better understand the mechanisms of corrosion and decomposition.
Essential Questionnaire
What is the difference between corrosion and decomposition?
Corrosion is a chemical process that occurs when a metal reacts with its environment, typically involving oxygen or water. Decomposition, on the other hand, is a broader term that encompasses the breakdown of organic materials into simpler substances.
What are the key factors that influence corrosion and decomposition?
Environmental factors such as temperature, humidity, and exposure to chemicals play a significant role in both corrosion and decomposition. Additionally, material properties, design, and maintenance practices can influence the susceptibility of materials to these processes.
How can we prevent corrosion and decomposition?
There are various methods to prevent corrosion and decomposition, including protective coatings, sacrificial anodes, and corrosion inhibitors. The choice of method depends on the specific material and environment.
