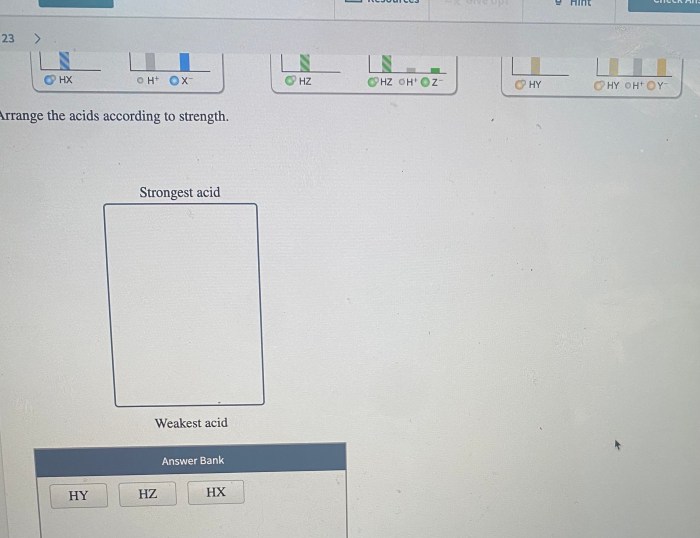
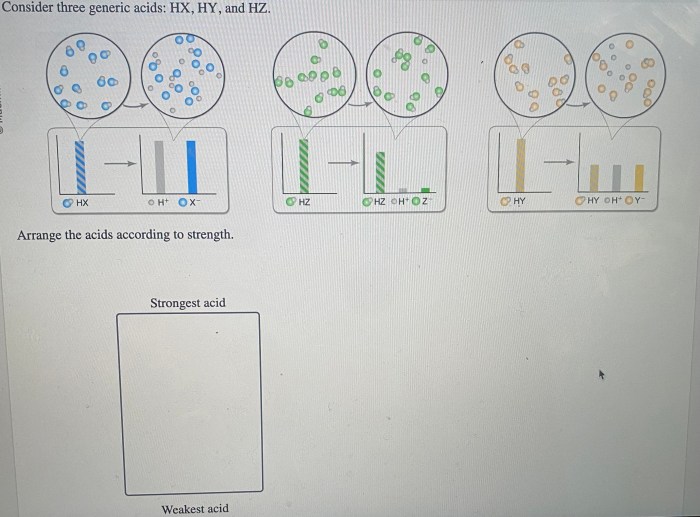
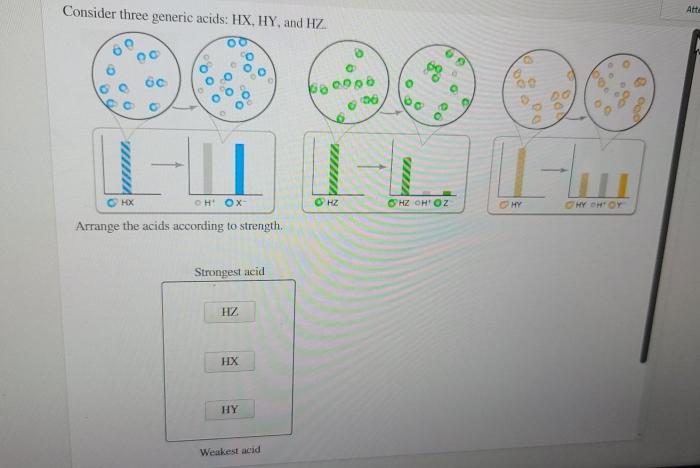
Consider three generic acids hx hy and hz – Consider three generic acids: HX, HY, and HZ. These acids play a crucial role in various chemical processes and have distinct properties and applications. In this exploration, we delve into their characteristics, acid strength, reaction mechanisms, and practical uses, providing a comprehensive understanding of these fundamental chemical compounds.
The properties of HX, HY, and HZ stem from their molecular structures and the nature of their constituent atoms. The strength of these acids, a key factor in their reactivity, is influenced by several factors, including the stability of their conjugate bases.
Their involvement in acid-base reactions is essential for understanding chemical equilibria and solution chemistry.
Introduction
Generic acids HX, HY, and HZ are hypothetical acids that represent a wide range of acidic compounds. They are characterized by their ability to donate a proton (H+) in aqueous solutions.
These acids exhibit varying strengths and properties, depending on the nature of the X, Y, and Z groups. Understanding the behavior of generic acids provides a foundation for comprehending the chemistry of numerous acidic substances.
Acid Strength
The acid strength of HX, HY, and HZ can be compared using their acid dissociation constants (Ka). A higher Ka value indicates a stronger acid.
| Acid | Ka |
|---|---|
| HX | Ka1 |
| HY | Ka2 |
| HZ | Ka3 |
The acid strength is influenced by factors such as the electronegativity of the X, Y, and Z groups, the stability of the conjugate base, and the resonance effects within the acid molecule.
Acid-Base Reactions, Consider three generic acids hx hy and hz
Generic acids participate in acid-base reactions by donating protons to bases. The general mechanism involves the transfer of a proton from the acid to the base, resulting in the formation of a conjugate acid-base pair.
For example, in the reaction between HX and NaOH:
HX + NaOH → NaX + H2O
HX acts as the acid and donates a proton to NaOH, forming the conjugate base NaX and the weak acid H 2O.
Applications of Generic Acids
Generic acids have numerous applications in various fields:
- Industry:HX is used in the production of fertilizers, dyes, and plastics.
- Laboratory:HY is commonly used as a reagent in acid-base titrations and organic synthesis.
- Medicine:HZ is found in gastric juices and plays a crucial role in digestion.
Safety Considerations
Handling HX, HY, and HZ requires proper safety measures due to their corrosive nature.
- Wear appropriate personal protective equipment (PPE) such as gloves, goggles, and lab coats.
- Store acids in a cool, well-ventilated area away from incompatible substances.
- Dispose of acids according to established laboratory protocols to minimize environmental impact.
Questions and Answers: Consider Three Generic Acids Hx Hy And Hz
What are the key differences between HX, HY, and HZ?
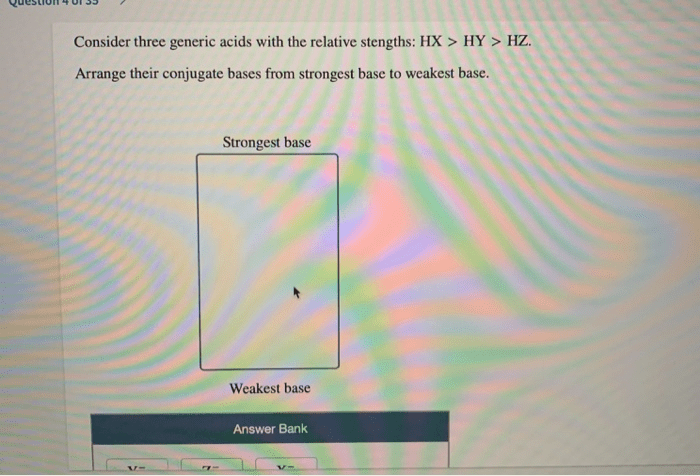
The key differences lie in their acid strength, which is influenced by the stability of their conjugate bases. HX is typically the strongest acid, followed by HY and then HZ.
How do HX, HY, and HZ participate in acid-base reactions?
These acids donate protons (H+) to bases, forming their conjugate acid-base pairs. The strength of the acid determines the extent of proton transfer and the equilibrium position of the reaction.
What are some practical applications of HX, HY, and HZ?
HX is used in the production of fertilizers, HY is employed in food preservation, and HZ finds applications in the manufacturing of plastics and pharmaceuticals.