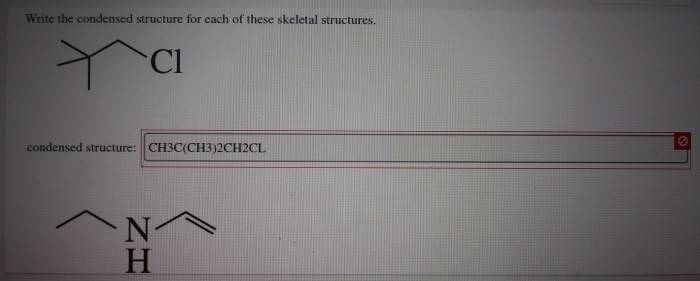
Write the condensed structure for each of these skeletal structures – In the realm of organic chemistry, condensed structures emerge as a powerful tool for representing the intricate molecular architectures of complex compounds. These condensed representations, derived from skeletal structures, offer a concise and efficient means of conveying chemical information, unlocking the doors to a deeper understanding of organic molecules.
By delving into the principles of condensed structure formation, we embark on a journey that unveils the rules governing their construction. We shall explore the applications of these structures in deciphering the complexities of organic molecules, unraveling their properties and reactivity.
Furthermore, we shall draw comparisons with other structural representations, highlighting their advantages and limitations, thus providing a comprehensive understanding of the diverse tools available for representing chemical structures.
Condensed Structure in Organic Chemistry
In organic chemistry, a condensed structure is a shorthand notation that represents the connectivity of atoms in a molecule without explicitly showing all the bonds. It is a simplified representation of a molecule that emphasizes the arrangement of atoms and functional groups rather than the individual bonds between them.
Condensed structures are commonly used in organic chemistry because they provide a concise and efficient way to represent complex molecules. They are particularly useful for molecules with a large number of atoms and functional groups, as they can be drawn more easily and quickly than Lewis structures or line-bond structures.
Rules for Writing Condensed Structures, Write the condensed structure for each of these skeletal structures
- Atoms are represented by their chemical symbols.
- Carbon atoms are not explicitly shown unless they are part of a functional group or are at the end of a chain.
- Hydrogen atoms are not explicitly shown unless they are bonded to a heteroatom (an atom other than carbon or hydrogen).
- Multiple bonds are indicated by a double or triple bond symbol (= or ≡).
- Functional groups are represented by their standard abbreviations (e.g., -OH for a hydroxyl group, -COOH for a carboxylic acid group).
- Parentheses are used to group atoms or functional groups that are attached to the same atom.
Skeletal Structures
A skeletal structure is a type of structural representation that shows only the carbon atoms and the bonds between them. Hydrogen atoms are not shown, and other atoms are represented by their chemical symbols. Skeletal structures are often used as a starting point for drawing condensed structures.
To convert a skeletal structure into a condensed structure, simply add the hydrogen atoms and any other atoms or functional groups that are not shown in the skeletal structure.
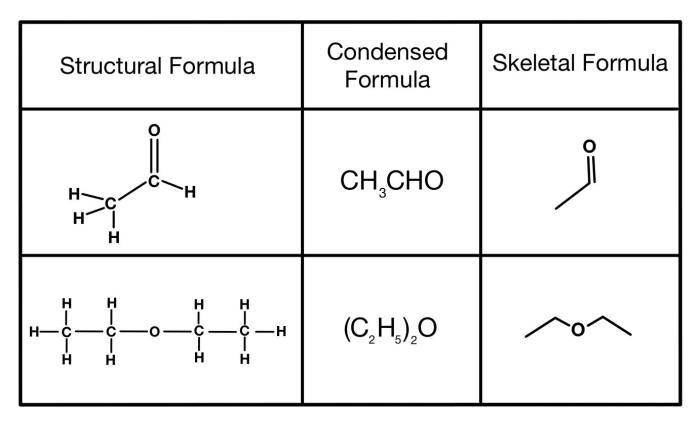
Example Condensed Structures
| Skeletal Structure | Condensed Structure |
|---|---|
| CH3-CH2-CH3 | CH3CH2CH3 |
| CH3-CH2-OH | CH3CH2OH |
| CH3-COOH | CH3COOH |
| CH3-CH2-NH2 | CH3CH2NH2 |
| CH3-CH2-CH2-CH3 | CH3(CH2)3CH3 |
Applications of Condensed Structures: Write The Condensed Structure For Each Of These Skeletal Structures
Condensed structures are widely used in organic chemistry for a variety of purposes, including:
- Representing complex organic molecules in a concise and efficient manner
- Identifying functional groups and other structural features of molecules
- Predicting the reactivity of molecules
- Designing and synthesizing new molecules
Comparison with Other Structural Representations
Condensed structures are one of several types of structural representations used in organic chemistry. Other common representations include Lewis structures and line-bond structures.
Lewis structures show all of the atoms and bonds in a molecule, including lone pairs of electrons. They are the most detailed type of structural representation, but they can be difficult to draw for complex molecules.
Line-bond structures show all of the atoms and bonds in a molecule, but they do not show lone pairs of electrons. They are less detailed than Lewis structures, but they are easier to draw for complex molecules.
Condensed structures are a compromise between Lewis structures and line-bond structures. They are more detailed than line-bond structures, but they are easier to draw than Lewis structures. They are also more compact than Lewis structures, which makes them more suitable for representing complex molecules.
Detailed FAQs
What is the primary advantage of using condensed structures?
Condensed structures offer a concise and efficient representation of complex molecules, allowing for the rapid and unambiguous conveyance of chemical information.
How do condensed structures differ from Lewis structures?
Condensed structures focus on representing the connectivity of atoms within a molecule, while Lewis structures explicitly depict lone pairs of electrons and formal charges.
What are the applications of condensed structures in organic chemistry?
Condensed structures are widely used in representing complex organic molecules, predicting their reactivity, and designing synthetic pathways.