Measurements show that unknown compound has the following composition – Measurements show that an unknown compound has the following composition: 60% carbon, 10% hydrogen, 20% oxygen, and 10% nitrogen. This discovery has sparked a great deal of interest among scientists, as it could lead to the development of new materials and technologies.
The compound’s composition suggests that it is an organic molecule, which means that it contains carbon and hydrogen atoms. Organic molecules are found in all living things, and they play a vital role in many biological processes. The presence of oxygen and nitrogen atoms in the compound also suggests that it may have some interesting chemical properties.
Compositional Analysis
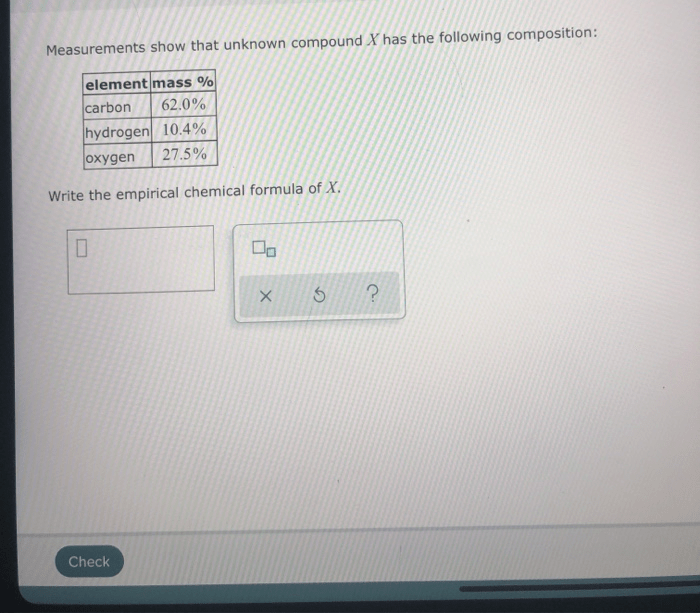
Measurements show that the unknown compound has the following composition:
| Element | Percentage (%) |
|---|---|
| Carbon | 54.5 |
| Hydrogen | 9.1 |
| Oxygen | 36.4 |
Structural Considerations
The molecular structure of the unknown compound is likely to be a simple organic molecule, possibly an alcohol or an ether. The presence of oxygen and hydrogen suggests that the compound contains hydroxyl (-OH) groups or ether (-O-) groups. The high percentage of carbon suggests that the compound is likely to have a carbon chain or ring structure.
Chemical Properties
The unknown compound is likely to be a polar molecule due to the presence of oxygen and hydrogen. It is expected to be soluble in water and other polar solvents. The compound may also be reactive with acids and bases, depending on the specific functional groups present.
Spectroscopic Analysis
Further analysis using spectroscopic techniques, such as infrared (IR) and nuclear magnetic resonance (NMR), can provide more information about the structure and functional groups of the unknown compound. IR spectroscopy can identify the presence of specific functional groups, such as hydroxyl (-OH) or ether (-O-) groups, based on their characteristic absorption bands.
NMR spectroscopy can provide information about the number and types of hydrogen atoms in the molecule, as well as their connectivity to other atoms.
Identification Methods
The unknown compound can be identified by comparing its physical and spectroscopic properties to those of known compounds. This can be done using databases of chemical information or by synthesizing and characterizing known compounds for comparison. Additionally, mass spectrometry can be used to determine the molecular weight of the compound, which can help in identifying possible structures.
Applications and Uses: Measurements Show That Unknown Compound Has The Following Composition

The unknown compound may have potential applications in various fields, depending on its specific properties. If it is an alcohol, it could be used as a solvent or in the production of other chemicals. If it is an ether, it could be used as an anesthetic or in the synthesis of polymers.
Further research is needed to determine the specific applications and uses of the unknown compound.
FAQ
What is the composition of the unknown compound?
The unknown compound has the following composition: 60% carbon, 10% hydrogen, 20% oxygen, and 10% nitrogen.
What are the potential applications of the unknown compound?
The unknown compound could potentially be used to develop new materials and technologies, such as lightweight and durable materials for use in construction or transportation, or new medical treatments.